About
The NHDR currently includes data from two longitudinal cohort studies.
Sickle Cell Disease Implementation Consortium (SCDIC)
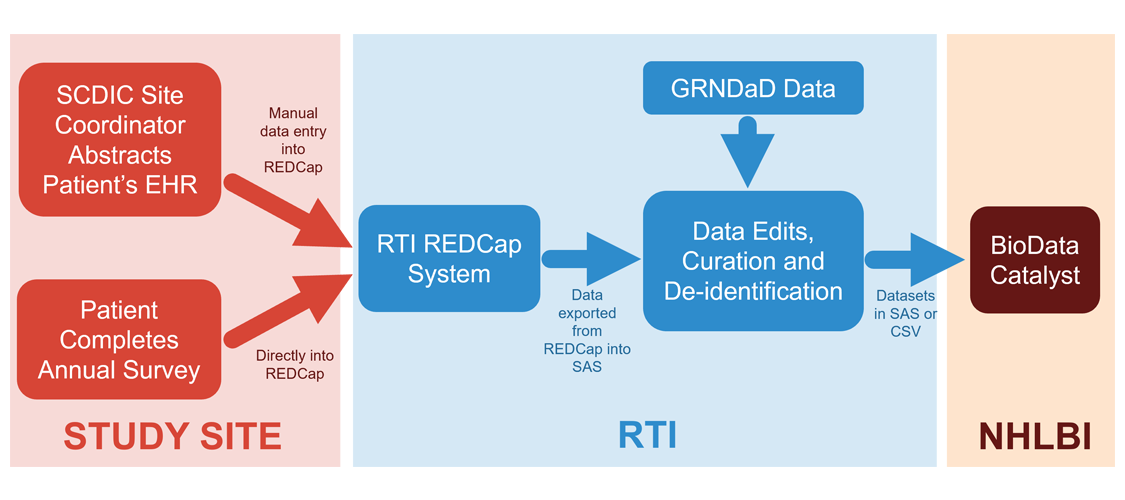
The SCDIC registry has been continuously funded by the NHLBI since 2016. Eight treatment centers across the United States participate in the SCDIC, with the support of RTI International as the Data Coordinating Center.
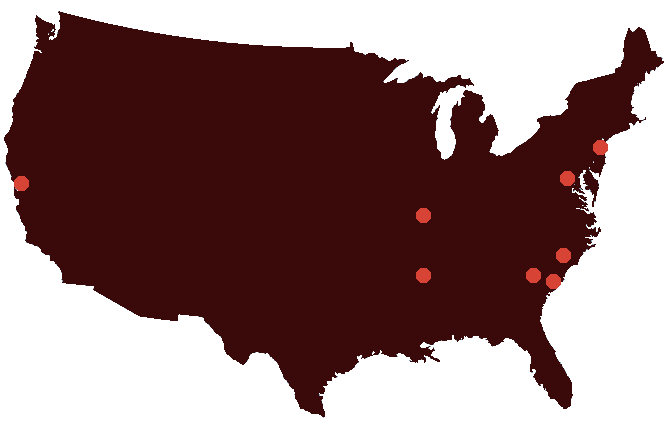

- Center: Augusta University (Augusta, GA)
- Center: University of Illinois at Chicago (Chicago, IL)
- Center: St. Jude Children's Research Hospital (Memphis, TN)
- Center: Washington University School of Medicine (St. Louis, MO)
- Center: Icahn School of Medicine at Mount Sinai (New York, NY)
- Center: Duke University (Durham, NC)
- Center: UCSF Benioff Children's Hospital Oakland (Oakland, CA)
- Center: Medical University of South Carolina (Charleston, SC)
- Coordinating Center: RTI International (Durham, NC)
- Program Agency: National Heart, Lung and Blood Institute (Bethesda, MD)
The SCDIC-I (2016-2022) enrolled over 2400 patients with SCD. Under the active SCDIC-II (2022-present), 1220 SCDIC-I patients were reconsented and an additional 450 new patients were enrolled from the same 8 centers between September 2022 and October 2023, for a total of 1670 in SCDIC-II. As a longitudinal study, each participant completes an annual survey and the study coordinators abstract an extensive set of data from the participant’s medical records. This provides a rich source of information over a period of many years, described in more detail here.
Characteristics of the SCDIC patient population
Age at Enrollment
| 14-25 | 1241 | (43.02%) |
| 26-35 | 996 | (34.52%) |
| 36-72 | 648 | (22.46%) |
Gender
| Female | 1639 | (56.81%) |
| Male | 1246 | (43.19%) |
Race
| Black | 2820 | (98.6%) |
| Other race | 40 | (1.4%) |
Inclusion/Exclusion Criteria
- SCDIC-I: 15-45 years at the time of consent
- SCDIC-II: At least 14 years of age at the time of consent
- Confirmed SCD diagnosis. Confirmed is defined as supported by documentation in the medical record of a positive test for one of the following: Hb SS, Hb SC, Hb Sβ-thalassemia, Hb SO, Hb SD, Hb SG, Hb SE, Hb SF
- Did not undergo a curative therapy for SCD
Globin Research Network for Data and Discovery (GRNDaD)
The GRNDaD Registry was selected to participate in the NHDR to enrich the repository with subjects who were enrolled in 2020 or earlier that were 8-20 years old at the time of enrollment. This includes a younger population that is not covered by the SCDIC. GRNDaD was initiated in 2017 and has enrolled over 1100 patients of all ages using a web-based REDCap data collection system. There are over 40 sites across the country, with Johns Hopkins University serving as the GRNDaD Data Coordinating Center. While GRNDaD includes patients recruited and consented at numerous SCD treatment centers across the United States, there are currently only two participating in the NHDR.
The image below shows how the data moves from collection to the data repository.